Isolation and Characterization of Staphylococcus spp. from a Plastic Recycling Plant and their Application in Mercury Wastewater Treatment
| Received 28 Jun, 2025 |
Accepted 01 Sep, 2025 |
Published 30 Sep, 2025 |
Background and Objective: Plastic recycling, while essential for sustainable waste management, can inadvertently lead to environmental pollution, including the release of heavy metals such as mercury, a highly toxic pollutant with serious health and ecological implications. Although Staphylococcusspp. are known for their diverse metabolic capabilities, their specific potential in mercury bioremediation within recycling environments remains largely unexplored. This study aims to isolate and characterize Staphylococcus species from a plastic recycling facility and evaluate their biosorption efficiency for mercury removal from contaminated wastewater. The influence of environmental factors, adsorption kinetics, and isotherm models was also investigated. Materials and Methods: Wastewater samples were collected from a local plastic recycling plant. Staphylococcus spp. were isolated using selective culture techniques and identified through morphological, biochemical, and molecular characterization. Batch biosorption experiments were conducted to evaluate the effects of pH (3-9), initial mercury concentration (5-50 mg/L), and contact time (up to 48 hrs). Mercury concentration was measured using atomic absorption spectrophotometry. The biosorption process was analyzed using Langmuir and Freundlich isotherms and pseudo-first- and second-order kinetic models. Regression analysis (R2) determined the best-fitting models. Results: Biosorption efficiency was highest (89.4%) at pH 7.0, with a maximum capacity of 18.9 mg/g at 25 mg/L mercury concentration. Removal peaked at 92.3% after 28 hours. The Freundlich isotherm model (R2 = 0.921) better described the adsorption behavior than the Langmuir model (R2 = 0.571), indicating multilayer adsorption. Kinetic modeling revealed the pseudo-second-order model (R2 = 0.935) as a better fit, suggesting chemisorption as the dominant mechanism. Conclusion: Staphylococcus spp. from plastic recycling wastewater exhibit high potential for mercury bioremediation. These findings support their application in developing eco-friendly, cost-effective treatment strategies. Future research should explore genetic mechanisms and field-scale implementation to enhance industrial applicability.
| Copyright © 2025 Anih et al. This is an open-access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
INTRODUCTION
Plastic recycling offers an effective approach to reducing environmental waste, yet it also presents potential environmental and health concerns, including microbial contamination and pollutant release¹. The exponential rise in plastic production has exacerbated the global plastic waste crisis, resulting in significant accumulation in landfills, oceans, and the broader environment2,3. Within recycling facilities, various microbial communities emerge due to the combination of organic waste, moisture, and fluctuating environmental conditions3. These microbes can originate from machinery, plastic materials, and environmental exposure. While some may aid recycling, others may produce harmful byproducts or pose health risks2.
Staphylococcus, a genus of Gram-positive cocci, is commonly found on human and animal skin and mucosal surfaces. It includes both pathogenic and non-pathogenic species. Pathogens like Staphylococcus aureus cause a range of diseases, whereas species such as S. epidermidis and S. saprophyticus are typically part of the normal flora4. Mercury, a highly toxic heavy metal, often enters ecosystems through mining, fossil fuel combustion, and industrial activities5. Its bioaccumulation in aquatic systems and toxicity to humans and wildlife necessitate urgent remediation strategies6.
Bioremediation utilizes microbial metabolism to detoxify or transform pollutants. Some Staphylococcal strains possess enzymes capable of degrading environmental toxins, including heavy metals like mercury7. Study reported microbial abilities to metabolize diverse chemicals for energy, highlighting bioremediation's eco-friendly potential8. However, limited data exist on isolating and characterizing Staphylococci from plastic recycling plants for mercury bioremediation9. This study addresses that gap by isolating and characterizing Staphylococcus strains from a recycling facility, assessing their mercury bioremediation capacity, and evaluating environmental factors influencing this ability. The study outcomes could inform environmental management, public health, and sustainable recycling practices10,11.
MATERIALS AND METHODS
Study area and sample collection: The research was conducted at the Microbiology Laboratory, Federal University Wukari, Taraba State, Nigeria, over six months (January-June, 2024). Soil samples were collected from a plastic recycling plant at Ambassador Roundabout, Wukari. Samples were placed in sterile containers and transported to the laboratory within 24 hrs12.
Materials: Materials used included hand gloves, cotton wool, Nutrient Agar (NA), MacConkey Agar (MA), Potato Dextrose Agar (PDA), petri dishes, sterile distilled water, wire loop, test tubes, autoclave, incubator, hydrogen peroxide, and Gram staining reagents13.
Media preparation: Culture media (NA, MA, and PDA) were prepared following manufacturer instructions and autoclaved at 121°C for 15 min at 15 psi. After sterilization, media were cooled to 50°C before use14,15.
Serial dilution: Five sterile test tubes were each filled with 9 mL distilled water and arranged in a tenfold serial dilution from 10 to 10 . One gram of soil was added to the first tube and mixed thoroughly. The 1 mL from each tube was transferred sequentially to the next and mixed16.
Plating and incubation: From appropriate dilutions, 1 mL was transferred to sterile petri dishes. Steri le media were poured into each dish, swirled, allowed to solidify, and incubated at 37°C for 24 hrs16.
Purification and storage of isolates: Distinct colonies were subcultured on fresh NA plates until pure isolates were obtained and maintained on slants at 4°C17.
Biochemical characterization
Gram staining: Bacterial smears were fixed and stained using crystal violet, iodine, acetone, and safranin18.
Catalase test: A H2O2 was dropped on a slide, and bacterial growth was emulsified. Bubble formation indicated a positive reaction19.
Coagulase test: Cultures were mixed with plasma in test tubes and incubated at 37°C. Clot formation indicated positivity20.
Oxidase test: Filter paper with bacterial growth was treated with oxidase reagent. A blue-black color within 30 sec indicated a positive result21.
TSI test: Isolates were inoculated into Triple Sugar Iron agar and incubated. Yellow color and/or black precipitate were interpreted22.
Citrate test: Simmons citrate agar was inoculated and incubated for 24 hrs. A blue color indicated citrate utilization23.
Indole test: Cultures in peptone water were incubated for 24 hrs. Indole reagent was added, and a red ring indicated a positive result24.
RESULTS AND DISCUSSION
Microbial count and colony characteristics: Plates from 10–3 to 10–5 dilutions showed distinct colonies. The average viable count was 4.2×105 CFU/g on Nutrient Agar25.
Gram reaction: All isolates were Gram-positive cocci, appearing in clusters suggestive of Staphylococcus spp26.
Catalase and coagulase test: All isolates were catalase-positive. Two out of ten isolates were coagulase-positive27.
Oxidase test: Six of ten isolates developed a blue-black color within 30 sec, indicating a positive oxidase reaction28.
Triple sugar iron (TSI) test: Four isolates fermented multiple sugars, turning the entire medium yellow. Three showed glucose-only fermentation (yellow butt, red slant), and three produced black precipitate (H2S production)29.
Simmons citrate test: Five isolates turned the medium blue, indicating citrate utilization; the remainder were citrate-negative30.
Indole test: Three isolates developed a red ring upon reagent addition (indole-positive), while seven showed no change (indole-negative)31.
Biosorption behavior and kinetic modeling of mercury removal by staphylococci
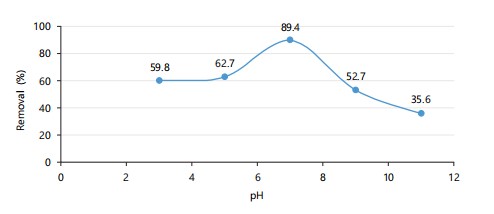
Effect of pH: The plot in Fig. 1 shows that mercury removal efficiency increases with pH and peaks at 89.4% at pH 7.0. This optimum is due to a balanced charge environment favoring ion exchange. Efficiency declines at extreme pH values because of proton competition and hydroxide precipitation.
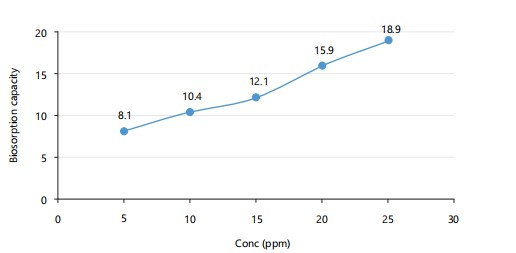
Effect of mercury concentration: As seen in Fig. 2, increasing mercury concentration leads to higher biosorption, with uptake reaching 18.9 mg/g at 25 ppm. The trend indicates more available ions and saturation of bacterial binding sites. Lower concentrations offer less driving force for adsorption.
|
|
|
|
|
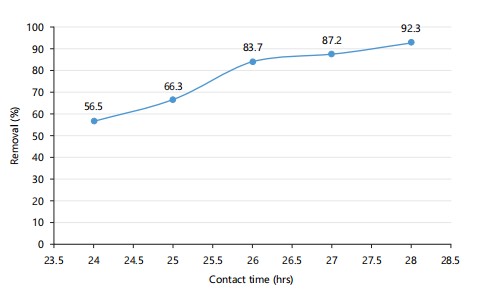
Effect of time: According to Fig. 3, biosorption improves as contact time extends from 24 to 28 hrs. This increase allows more mercury ions to bind to active sites on the bacterial surface. A plateau suggests equilibrium is approached at longer durations.
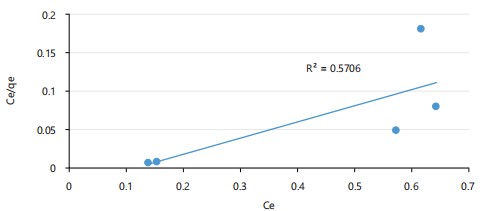
Langmuir isotherm model: The adsorption pattern presented in Fig. 4 corresponds to the Langmuir model, which assumes monolayer adsorption on uniform surfaces. The poor fit (R2 = 0.571) implies that this model is less appropriate for the studied system.
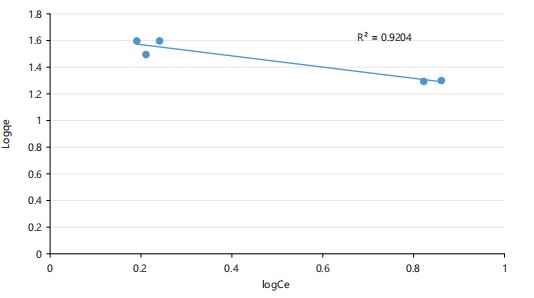
Freundlich isotherm model: As illustrated in Fig. 5, the Freundlich isotherm describes adsorption on heterogeneous surfaces with multilayer formation. The high correlation (R2 = 0.921) supports the suitability of this model for mercury biosorption by Staphylococci.
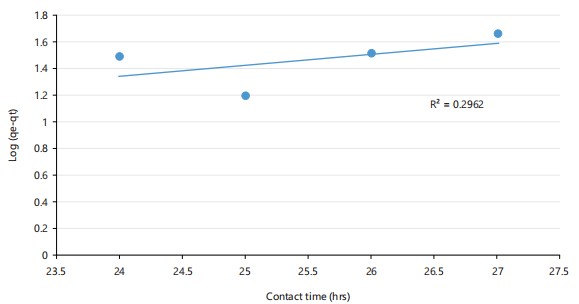
Pseudo-first order kinetic model: The linearity of the graph in Fig. 6 is weak, with an R2 of 0.296, indicating poor alignment between experimental and predicted values. This suggests that the pseudo-first order model does not adequately explain the adsorption kinetics in this case.
|
|
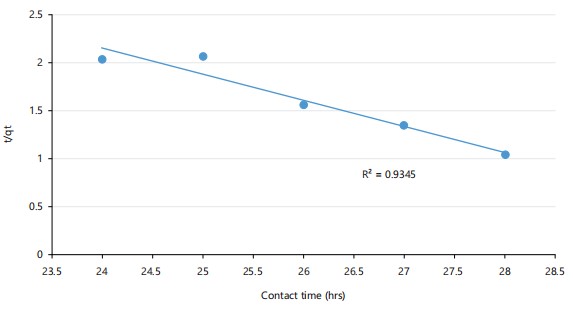
Pseudo-second order kinetic model: A stronger correlation in Fig. 7 (R2 = 0.935) confirms the pseudo-second order model best describes the kinetic data. This indicates that chemisorption, involving valence forces or electron sharing, governs mercury uptake by the bacteria.
Equilibrium isotherm parameters: Table 1 presents the equilibrium isotherm parameters for mercury biosorption by Staphylococci isolated from a plastic recycling plant.
| • | It compares the fit of the Langmuir and Freundlich isotherm models using constants and correlation coefficients | |
| • | The data highlight a better fit for the Freundlich model, indicating multilayer adsorption | |
| • | This suggests surface heterogeneity in the biosorption process32,33 |
| Table 1: | Equilibrium isotherm parameters for the biosorption of Hg(II) ions by Staphylococci isolated from a plastic recycling plant | |||
| Isotherm model | Hg(II) |
| Langmuir model | |
| qL (mg/g) | 10.013 |
| KL (L/mg) | 0.11 |
| R2 | 0.571 |
| Freundlich model | |
| KF (L/g) | 1.02 |
| 1/n | 0.301 |
| R2 | 0.921 |
| Equilibrium isotherm constants for Hg(II) ion biosorption, Freundlich model showed higher correlation (R2 = 0.921) than Langmuir (R2 = 0.571) and data support heterogeneous, multilayer biosorption behavior | |
| Table 2: | Kinetic parameters for the biosorption of Hg(II) ions by Staphylococci isolated from a plastic recycling plant | |||
| Kinetic models | Hg(II) |
| qeexp (mg/g) | 28.83 |
| Pseudo-first order | |
| qecal (mg/g) | 8.214 |
| K1 (min) | 0.131 |
| R2 | 0.296 |
| Pseudo-second order | |
| qecal (mg/g) | 25.21 |
| K2 (g/mg/min) | 0.15 |
| h (mg/g/min) | 1.121 |
| R2 | 0.935 |
| Kinetic model parameters for Hg(II) ion biosorption, pseudo-second-order model showed superior fit (R2 = 0.935) and results confirm chemisorption governs the biosorption kinetics34,35 | |
Kinetic model parameters: Table 2 details kinetic parameters for the biosorption of Hg(II) by Staphylococci.
| • | It compares pseudo-first-order and pseudo-second-order kinetic models using calculated adsorption capacities and correlation coefficients | |
| • | The pseudo-second-order model exhibited a higher correlation coefficient and better fit | |
| • | This indicates chemisorption as the dominant biosorption mechanism32,33 |
This study demonstrated that Staphylococcus spp., isolated from a plastic recycling facility, possess substantial potential for mercury biosorption from contaminated wastewater. The maximum removal efficiency reached 89.4% at neutral pH, with a biosorption capacity of 18.9 mg/g at a mercury concentration of 25 ppm. These results align with recent concerns raised by Singh and Walker1, who emphasized that plastic recycling, while beneficial for waste management, can also contribute to environmental contamination if pollutant mitigation is not incorporated into the system design. They advocate for integrated approaches that utilize eco-friendly, in-situ solutions such as microbial remediation to address pollution within recycling operations.
The role of pH in biosorption efficiency was found to be critical, with optimal mercury uptake occurring at pH 7.0. This pH likely enhances electrostatic interactions between the negatively charged bacterial surfaces and positively charged mercury ions. A similar observation was reported by Zhang et al.15, who explained that biosorption processes are strongly influenced by the ionization state of functional groups and the chemical form of heavy metals in solution, both of which are modulated by pH. At acidic conditions (pH 3.0), reduced biosorption was likely due to competition between protons and metal ions for binding sites, whereas at alkaline pH (9.0-11.0), mercury ions may have precipitated as hydroxides, leading to lower bioavailability for adsorption. This behavior is consistent with findings by Xia et al.26, who demonstrated similar pH-dependent mechanisms in their study on mercury adsorption.
An increase in initial mercury concentration led to higher biosorption capacity, though the percentage removal plateaued. This pattern suggests a saturation effect due to the finite number of active sites on bacterial surfaces. Naseem and coworkers observed a comparable trend in their study using Sorghum bicolor biomass, highlighting that biosorbent saturation becomes evident at elevated contaminant loads.
Contact time also influenced biosorption, with peak mercury removal (92.3%) occurring after 28 hrs. This suggests a two-phase kinetic process involving rapid initial adsorption followed by slower diffusion or chemical interactions. Such biphasic kinetics are widely reported in biosorption studies. For example, Olasehinde et al.35 described a similar time-dependent removal pattern using Raphia taedigera-based activated carbon for dye biosorption. In this study, the data conformed better to the pseudo-second-order kinetic model (R2 = 0.935) than the pseudo-first-order model (R2 = 0.296), indicating chemisorption as the predominant mechanism. Bullen and collaborators32 proposed an improved kinetic model sensitive to adsorbate and adsorbent concentrations, reinforcing the utility of pseudo-second-order kinetics for accurately modeling chemisorption processes.
Adsorption isotherm modeling revealed that the Freundlich model (R2 = 0.921) described the biosorption behavior more accurately than the Langmuir model (R2 = 0.571). This suggests that biosorption occurred on a heterogeneous surface with varying affinities and multilayer adsorption. Chakraborty et al.31 previously reported similar findings in their review of microbial biosorbents, underscoring the complexity of biological surfaces rich in proteins, teichoic acids, and polysaccharides.
The effectiveness of Staphylococcus spp. in mercury biosorption is supported by other studies on microbial metal remediation. Aranda and Rivas, for instance, outlined the proficiency of bacterial bioadsorbents in sequestering toxic metals such as mercury and lead, emphasizing their relevance in wastewater treatment technologies. Similarly, El-Sharkawy et al.29 demonstrated enhanced lead biosorption using Bacillus subtilis, which reinforces the applicability of bacterial strains for heavy metal removal under optimized conditions.
The localized sourcing of functional biosorbents from within the pollution site itself, as demonstrated in this study, supports the principle of system-specific remediation strategies. Singh and Walker1 stressed the importance of such approaches within the framework of the circular economy, arguing that in-situ microbial solutions can reduce environmental impact and contribute to closed-loop sustainability in industrial systems.
Nonetheless, certain limitations must be acknowledged. The experiments were conducted in controlled laboratory settings that may not capture the complexity of real-world industrial wastewater, which typically contains multiple co-contaminants. Furthermore, only a single bacterial genus was explored. Future studies should investigate microbial consortia or biofilm-based systems to assess potential synergistic effects. To better understand the mechanisms involved, advanced molecular diagnostic tools such as 16S ribosomal ribonucleic acid (16S rRNA) gene sequencing, as described by Li et al.4, could be employed to identify biosorption-related genes and enhance strain selection through targeted genetic or metabolic engineering.
CONCLUSION
This experimental investigation demonstrated the potential of Staphylococcus spp. isolated from a plastic recycling plant as an effective biosorbent for Mercury removal from aqueous solutions with a peak biosorption efficiency of 89.4% at neutral pH (7.0) and a biosorption capacity of 19.7 mg/g. Low pH reduced biosorption due to proton competition, while high pH led to mercury precipitation. The biosorption capacity also increased with increasing mercury concentrations, ranging from 8.1 mg/g at 5 ppm to 18.9 mg/g at 25 ppm. As mercury concentrations increased, the final absorbance values fell, indicating a greater degree of mercury removal from the solution. The study reveals pH plays a significant role in biosorption efficiency. The experimental data suggested that mercury biosorption followed the Langmuir isotherm, indicating monolayer adsorption onto a homogeneous surface. The biosorption process followed pseudo-second-order kinetics, implying that chemisorption (chemical interactions) played a major role rather than just physical adsorption. These findings confirm that Staphylococcus spp. is a promising biosorbent for mercury remediation in wastewater treatment, particularly under neutral pH conditions, making it economical and sustainable alternative to normal mercury removal methods.
SIGNIFICANCE STATEMENT
This study provides novel insights into the use of Staphylococcus species, isolated from a plastic recycling plant, as effective biosorbents for the remediation of mercury-contaminated wastewater. In an era where plastic recycling is both a solution to waste management and a source of environmental pollutants, this research addresses a critical intersection of microbiology, environmental biotechnology, and public health. The research demonstrates, for the first time, the capacity of indigenous Staphylococci to remove up to 92.3% of mercury under optimized conditions, with biosorption kinetics revealing a chemisorption-dominated mechanism best described by the pseudo-second-order model. The findings not only advance our understanding of microbial biosorption processes but also highlight the untapped potential of bacteria in industrial waste environments as cost-effective, sustainable, and eco-friendly agents for heavy metal remediation. This work lays the foundation for developing integrated bioremediation strategies within the framework of circular economy practices in the recycling industry.
ACKNOWLEDGMENT
The authors gratefully acknowledge the Department of Biochemistry, Faculty of Biosciences, Federal University Wukari, Taraba State, Nigeria, for providing the laboratory facilities and technical support necessary for the successful completion of this research. Special thanks go to the staff of the Microbiology Laboratory for their assistance during the microbial analysis and batch adsorption experiments. We also extend our appreciation to the management of the plastic recycling facility in Wukari for granting access to sample collection.
REFERENCES
- Singh, N. and T.R. Walker, 2024. Plastic recycling: A panacea or environmental pollution problem. npj Mater. Sustainability, 2.
- Khajvand, M., A.K. Mostafazadeh, P. Drogui and R.D. Tyagi, 2022. Management of greywater: Environmental impact, treatment, resource recovery, water recycling, and decentralization. Water Sci. Technol., 86: 909-937.
- Bisht, A., N. Kamboj, V. Kamboj and A. Bisht, 2020. A review on the role of emerging anthropogenic activities in environmental degradation and emphasis on their mitigation. Arch. Agric. Environ. Sci., 5: 419-425.
- Li, M.N., Q. Han, N. Wang, T. Wang and X.M. You et al., 2024. 16S rRNA gene sequencing for bacterial identification and infectious disease diagnosis. Biochem. Biophys. Res. Commun., 739.
- Han, D., F. Yu, D. Zhang, J. Hu and X. Zhang et al., 2024. Molecular rapid diagnostic testing for bloodstream infections: Nanopore targeted sequencing with pathogen-specific primers. J. Infect., 88.
- Chhetri, S., M.T. Sherpa and L. Sharma, 2025. Characterization of plant growth promoting bacteria isolated from rhizosphere of tomato cultivated in Sikkim Himalaya and their potential use as biofertilizer. Sci. Rep., 15.
- Hussain, A., S. Madan and R. Madan, 2021. Removal of Heavy Metals from Wastewater by Adsorption. In: Heavy Metals-Their Environmental Impacts and Mitigation, Nazal, M.K. and H. Zhao (Eds.), IntechOpen, London, United Kingdom, ISBN: 978-1-83968-122-6 .
- Melhi, S., S.U. Jan, A.A. Khan, K. Badshah, Saeed Ullah, B. Bostan and Z. Selamoglu, 2022. Remediation of Cd (II) ion from an aqueous solution by a starch-based activated carbon: Experimental and density functional theory (DFT) approach. Crystals, 12.
- Patel, H., 2022. Comparison of batch and fixed bed column adsorption: A critical review. Int. J. Environ. Sci. Technol., 19: 10409-10426.
- Aziam, R., D.S. Stefan, S. Nouaa, M. Chiban and M. Boșomoiu, 2024. Adsorption of metal ions from single and binary aqueous systems on bio-nanocomposite, alginate-clay. Nanomaterials, 14.
- Bakr, A.A., N.A. Sayed, T.M. Salama, I.O. Ali, R.R. Abdel Gayed and N.A. Negm, 2018. Kinetics and thermodynamics of Mn(II) removal from aqueous solutions onto Mg-Zn-Al LDH/montmorillonite nanocomposite. Egypt. J. Pet., 27: 1215-1220.
- Neag, E., A.I. Török, C. Tanaselia, I. Aschilean and M. Senila, 2020. Kinetics and equilibrium studies for the removal of Mn and Fe from binary metal solution systems using a Romanian thermally activated natural zeolite. Water, 12.
- Park, S., J.W. Lee, J.E. Kim, G. Kang, H.J. Kim, Y.K. Choi and S.H. Lee, 2022. Adsorptive behavior of Cu2+ and benzene in single and binary solutions onto alginate composite hydrogel beads containing pitch pine-based biochar. Polymers, 14.
- Al-Ghouti, M.A. and D.A. Da'ana, 2020. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater., 393.
- Zhang, X., R. Shan, X. Li, L. Yan, Z. Ma, R. Jia and S. Sun, 2021. Effective removal of Cu(II), Pb(II) and Cd(II) by sodium alginate intercalated MgAl-layered double hydroxide: Adsorption properties and mechanistic studies. Water Sci. Technol., 83: 975-984.
- Aranda, F.L. and B.L. Rivas, 2022. Removal of mercury and lead by bioadsorbents. An overview. J. Chil. Chem. Soc., 67: 5682-5691.
- Tewari, A., D.S. Bhutada and V. Wadgaonkar, 2023. Heavy metal remediation from water/wastewater using bioadsorbents-A review. Nat. Environ. Pollut. Technol., 22: 2039-2053.
- Dias, M., J. Pinto, B. Henriques, P. Figueira and E. Fabre et al., 2021. Nutshells as efficient biosorbents to remove cadmium, lead, and mercury from contaminated solutions. Int. J. Environ. Res. Public Health, 18.
- Shamim, S., 2018. Biosorption of Heavy Metals. In: Biosorption, Derco, J. and B. Vrana, IntechOpen, London, United Kingdom, ISBN: 978-1-78923-473-2, pp: 21-49.
- Jitjaroendee, T., S. Chanmungkalakul, V. Ervithayasuporn and S. Kiatisevi, 2025. Silica-based materials for mercury detection and removal: A chelation-free solution. Chem. Asian J., 20.
- Tounsi, A., I. Nouacer, S. Hammad, M. Benalia and M. Djedid, 2023. Kinetics, thermodynamic, and isotherm modeling for biosorption of heavy metals from aqueous environment onto lignocellulosic biomass. Iran. J. Chem. Chem. Eng., 42: 1784-1795.
- Fabre, E., C. Vale, E. Pereira and C.M. Silva, 2021. Sustainable water treatment: Use of agricultural and industrial wastes to remove mercury by biosorption. Water Air Soil Pollut., 232.
- Boakye, P., G. Ohemeng-Boahen, L. Darkwah, Y.A. Sokama-Neuyam and E. Appiah-Effah et al., 2022. Waste biomass and biomaterials adsorbents for wastewater treatment. Green Energy Environ. Technol., 2022.
- Min, H.S. and S. Ray, 2024. Removal of mercury Ions from wastewater using different techniques. Int. J. Eng. Trends Technol., 72: 21-34.
- Zhang, Y., 2025. A review on the mechanism and influencing factors of heavy metal removal by biosorption method. J. Geosci. Environ. Prot., 13.
- Xia, M., Z. Chen, Y. Li, C. Li, N.M. Ahmad, W.A. Cheema and S. Zhu, 2019. Removal of Hg(II) in aqueous solutions through physical and chemical adsorption principles. RSC Adv., 9: 20941-20953.
- Sharma, S., A. Hasan, N. Kumar and L.M. Pandey, 2018. Removal of methylene blue dye from aqueous solution using immobilized Agrobacterium fabrum biomass along with iron oxide nanoparticles as biosorbent. Environ. Sci. Pollut. Res., 25: 21605-21615.
- Dalal, S.R., 2025. Efficacious biosorption of crystal violet pollutant dye from aqueous solutions via Padina pavonica derived alginate. Sci. Rep., 15.
- El-Sharkawy, R.M., M. Khairy, M.H.H. Abbas, M.E.A. Zaki and A.E. El-Hadary, 2024. Innovative optimization for enhancing Pb2+ biosorption from aqueous solutions using Bacillus subtilis. Front. Microbiol., 15.
- Obayomi, K.S., M. Auta and A.S. Kovo, 2020. Isotherm, kinetic and thermodynamic studies for adsorption of lead(II) onto modified Aloji clay. Desalin. Water Treat., 181: 376-384.
- Chakraborty, R., A. Asthana, A.K. Singh, B. Jain and A.B.H. Susan, 2022. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem., 102: 342-379.
- Liu, C., C. Wang, M. Chen, H. Hu and Q. Zhang, 2021. Mechanochemical disproportionation reaction of sulfur on Bi2O3 to synthesize Bi2O2S for simultaneous removals of Cu2+ and Cl- from waste solution. J. Environ. Chem. Eng., 9.
- Bai, L., X. Su, J. Feng and S. Ma, 2021. Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr (VI) adsorption in water. J. Cleaner Prod., 320.
- Naseem, K., Z.H. Farooqi, M.Z. Ur Rehman, M.A. Ur Rehman and R. Begum et al., 2018. A systematic study for removal of heavy metals from aqueous media using Sorghum bicolor: An efficient biosorbent. Water Sci. Technol., 77: 2355-2368.
- Olasehinde, E.F., S.M. Abegunde and M.A. Adebayo, 2020. Adsorption isotherms, kinetics and thermodynamic studies of methylene blue dye removal using Raphia taedigera seed activated carbon. Caspian J. Environ. Sci., 18: 329-344.
How to Cite this paper?
APA-7 Style
Anih,
D.C., Tatah,
S.V., Boyi,
R.N., Yohanna,
E.R., Abu,
M.S., Shadrach,
P., Mgbede,
T., Enenche,
M.O. (2025). Isolation and Characterization of Staphylococcus spp. from a Plastic Recycling Plant and their Application in Mercury Wastewater Treatment. Trends in Environmental Sciences, 1(3), 307-317. https://doi.org/10.21124/tes.2025.307.317
ACS Style
Anih,
D.C.; Tatah,
S.V.; Boyi,
R.N.; Yohanna,
E.R.; Abu,
M.S.; Shadrach,
P.; Mgbede,
T.; Enenche,
M.O. Isolation and Characterization of Staphylococcus spp. from a Plastic Recycling Plant and their Application in Mercury Wastewater Treatment. Trends Env. Sci 2025, 1, 307-317. https://doi.org/10.21124/tes.2025.307.317
AMA Style
Anih
DC, Tatah
SV, Boyi
RN, Yohanna
ER, Abu
MS, Shadrach
P, Mgbede
T, Enenche
MO. Isolation and Characterization of Staphylococcus spp. from a Plastic Recycling Plant and their Application in Mercury Wastewater Treatment. Trends in Environmental Sciences. 2025; 1(3): 307-317. https://doi.org/10.21124/tes.2025.307.317
Chicago/Turabian Style
Anih, David, Chinonso, Silas Verwiyeh Tatah, Richard-Harris Nsenreuti Boyi, Emochone Roy Yohanna, Michael Sunday Abu, Phillip Shadrach, Timothy Mgbede, and Marypeace Omega Enenche.
2025. "Isolation and Characterization of Staphylococcus spp. from a Plastic Recycling Plant and their Application in Mercury Wastewater Treatment" Trends in Environmental Sciences 1, no. 3: 307-317. https://doi.org/10.21124/tes.2025.307.317

This work is licensed under a Creative Commons Attribution 4.0 International License.




